
SMOKE, BUT NO FIRE
Tiny little discoloration features in mica and other minerals are believed by many creationists to be the "smoking gun" proof of a young Earth.
Thomas A. Baillieul, Columbus, Ohio
Last update, October, 2008
Introduction
As the creation/evolution debate continues, there has been an increasing sophistication of certain Creationist arguments and publications. It can be an especially difficult challenge when the Creationist author has professional credentials and has published in mainstream scientific journals. One such individual is Robert Gentry, who holds a Master's degree in Physics (and an honorary doctorate from the fundamentalist Columbia Union College). For over thirteen years he held a research associate's position at the Oak Ridge National Laboratory where he was part of a team which investigated ways to immobilize nuclear waste. Gentry has spent most of his professional life studying the nature of very small discoloration features in mica and other minerals, and concluded that they are proof of a young Earth.

Figure 1. Radiation damage haloes around zircon inclusions in pyroxene (160X magnification). Authors collection.
To fully understand Gentry's hypothesis a basic background in geology, mineralogy, and radiation physics is helpful. The boxes on the next few pages present a brief tutorial in rocks, minerals, and radioactivity. Certain minerals, such as zircon and monazite, which form as common trace constituents in igneous rocks, have crystal structures which can accommodate varying amounts of the naturally occurring radioactive elements, uranium and thorium. When these minerals occur as inclusions in certain other minerals, most notably the mica family, they are often seen to develop discoloration, or "pleochroic" haloes. The haloes are caused by radiation damage to the host mineral's crystalline structure. Figure 1 shows a typical discoloration halo around a radioactive mineral inclusion in the mineral pyroxene. The zone of damage is roughly spherical around a central mineral inclusion or radioactive source. Note that the halo has the highest intensity of discoloration near the source, gradually fading with distance in the host mineral to a "fuzzy" edge.
Radiation damage haloes around mineral inclusions are well known from the geological literature. Discoloration haloes in younger rocks tend to be smaller and less intense than in older rocks, indicating that the zone of crystal damage increases with time. From these observations early attempts were made to use the dimensions of haloes as an age dating technique. This was never fully successful as the size/intensity of an observed damage halo was also a function of the abundance of radionuclides present in the inclusion, and the crystalline structure of the host mineral.
| About the Rocks
Geologists classify rocks into three main categories - sedimentary, igneous, and metamorphic - based on the way in which they form. Sedimentary rocks are secondary in formation, being derived from precursor rocks (of any type).
Igneous rocks form from molten material, and are further subdivided into two main categories, the volcanic rocks which form from lava extruded at or near the surface; and plutonic rocks which form from magma, intruded deep within the crust. Both types of igneous rocks comprise a mixture of different minerals. As igneous rocks cool, mineral crystals form following a specific sequence. The crystals develop an interlocking texture with some of the trace minerals becoming completely surrounded by later forming crystals. Volcanic rocks, because they cool and crystalize rapidly, have a very fine-grained texture; the individual mineral grains are too small to see easily with the naked eye. Plutonic rocks on the other hand cool very slowly, on the order of a million years or more for some deeply buried and insulated magmas. The mineral grains in these rocks can grow very large and are readily distinguished in hand samples. Granite is a well-known type of plutonic igneous rock, but there are many others as well. Geologists distinguish these types of rock based on their chemical and mineralogical composition. Granites, for example, have more than 10% quartz and abundant potassium feldspar. Other plutonic rocks have less quartz and potassium, and different ratios of calcium and sodium feldspar (a common rock-forming mineral). True granites are relative latecomers on the geologic scene as they required a number of recycles of crustal material to differentiate and concentrate potassium. Lorence Collins (1999) provides a thorough overview of the origin and nature of granitic rocks. Metamorphic rocks represent alterations of precursor sedimentary, igneous, or other metamorphic rocks. Through the cycles of burial, folding, faulting, and subduction of crustal plates, rocks get pushed and dragged down to depths where - under heat and pressure - changes take place. In metamorphic rocks, new minerals form that are more stable at higher temperatures and pressures. Sometimes the minerals segregate into distinct bands. When burial pressures and temperatures get too great, the rocks melt completely, becoming new igneous rocks. |
|---|
Gentry's thesis has several components. First is his contention that the granitic rocks from which samples reportedly came constitute the "primordial" crust of the Earth. Within these rocks are biotite (an iron-bearing form of mica) and fluorite crystals which bear a relatively uncommon class of tiny, concentric discoloration "haloes" (figure 2). These haloes were considered to be the result of damage to the crystal structure of the host minerals caused by high energy alpha particles. In numerous papers published in scientific journals in the 1970s and 1980s, Gentry built the case that the different alpha decay energies of various naturally occurring radioactive isotopes resulted in distinctly different halo diameters. Thus, Gentry concluded that he could distinguish haloes resulting uniquely from the radioactive decay of various isotopes of the element polonium. Polonium, part of the decay chain of natural uranium and thorium, has a very short half-life - measured in microseconds to days, depending on the specific isotope. Concentric haloes associated with polonium decay - but without any rings corresponding to any other uranium decay series isotopes were taken to be evidence that the host rock had formed almost instantly rather than by the slow cooling of an original magma over millions of years. Gentry extrapolates that all Precambrian granites - his primordial crustal rock - must have formed in less than three minutes, and that polonium haloes are therefore proof of the young Earth creation model according to Genesis.
| Radioactivity
Radioactivity is a phenomenon of the nucleus of atoms. You may recall from high school chemistry class that an atom is composed of: protons, which carry a positive charge; neutrons, with no charge; and negatively charged electrons. The protons and neutrons together form the nucleus of the atom, surrounded by a swarm of electrons in distinct orbits. In neutral atoms, the numbers of protons and electrons always match, their charges balancing. It is the number of protons (and hence the number of electrons) that give an element its unique chemical characteristics.
Atoms, however, can have different numbers of neutrons without changing their chemical behavior. For example, the simplest atom, hydrogen, has one proton and one electron. Two additional varieties of hydrogen exist: one which has one neutron in addition to the proton (called deuterium); and one with two neutrons (known as tritium). Different varieties of the same element are known as isotopes. Uranium has 92 protons, but has different isotopes with 141, 142, 143, 144, 145, and 146 neutrons. Radioactivity is a complex phenomenon, but it can be thought of simply as the consequence of the imbalance caused in an atomic nucleus by an over abundance of neutrons. Isotopes which have too many neutrons try to become more stable by getting rid of neutrons through a number of means, the most common being the emission of high energy alpha and beta particles. An alpha particle comprises two protons and two neutrons, and is chemically indistinguishable from a helium nucleus [as a matter of fact, all the helium gas sold commercially comes from the radioactive decay of uranium, the gas occasionally being trapped in oil deposits that overlie uranium ore bodies]. Emission of an alpha particle creates a new chemical element with two less protons than its parent atom. The radioactive isotope Uranium-238 (92 protons) decays by giving off an alpha particle to become an atom of Thorium-234 (90 protons). Beta particles are created when a neutron breaks down into a proton and an electron - the beta particle thus is an electron, only in this case it comes from the nucleus. In beta decay, the proton remains in the nucleus, also causing the atom to adopt a new chemical identity. Rubidium-87 (37 protons) decays to become Strontium-87 (38 protons). Other types of radioactive decay schemes are known to exist, but are much less common than alpha and beta particle emission - and don't really play in the subject at hand. One last point - radioactivity is a statistical phenomenon. Not all the radioactive atoms within a mass decay at the same time. For example, an amount of uranium-238 decays at a rate such that after 4.5 billion years half of the original mass has been converted to other atoms. Several of the "daughter" atoms in the decay series of uranium-238 are themselves radioactive and decay at their own statistical rates until eventually the stable, non-radioactive isotope of Lead-206 is reached. |
|---|
For this hypothesis to be accepted, it must be testable. Fortunately, Gentry's thesis allows us to pose several questions which can be answered by looking at the evidence from the natural world. A yes answer to each question would significantly strengthen Gentry's arguments.
1) Do the rocks from which Gentry drew his samples represent the "primordial" basement rocks of the originally created Earth?
Gentry is a physicist, not a geologist. He doesn't follow accepted geologic reporting practice and consistently fails to provide the information that a third party would need to collect comparable samples for testing. For his research, Gentry utilized microscope thin sections of rocks from samples sent to him by others from various places around the world. Thus, he is unable to say how his samples fit in with the local or regional geological setting(s). He also does not provide descriptive information about the individual rock samples that make up his studies - i.e., the abundance and distribution of major, accessory, or trace minerals; the texture, crystal size and alteration features of the rocks; and the presence or absence of fractures and discontinuities.
Gentry does not acknowledge that the Precambrian time period represents fully 7/8 of the history of the Earth as determined by decades of intensive field and laboratory investigations by thousands of geologists. Consequently, he does not recognize the wide diversity of geologic terranes that came and went over that enormous time span. His claim that his samples represent "primordial" basement rocks is patently incorrect . In Gentry's model, any rock looking vaguely like a granite and carrying the label Precambrian is considered to be a "primordial" rock. True granites are themselves evidence of significant crustal recycling and elemental differentiation (see for example, Taylor and McLennan, 1996), and cannot be considered primordial. A little detective work by Wakefield (1988) showed that at least one set of rock samples studied by Gentry are not from granites at all, but were taken from a variety of younger Precambrian metamorphic rocks and pegmatite veins in the region around Bancroft, Ontario. Some of these rock units cut or overlie older, sedimentary and even fossil-bearing rocks.
Gentry provides no explanation for how polonium alone finds its way into biotite and fluorite, or why radiation damage haloes in these minerals are common in areas of known uranium enrichment, but rare where uranium abundance is low. Gentry's hypothesis would seem to suggest that there should be a uniform distribution of all polonium isotopes in primordial rocks, or at least no particular spatial association with uranium. Gentry (1974), himself, notes that haloes have not been found in meteorites or lunar samples, rocks known to be very low in uranium abundance. Lorence Collins (1997) has noted these and several other contradictory situations between the polonium halo hypothesis and observed geological relationships in the field.
2) Are the concentric haloes observed by Gentry actually caused by alpha particle damage to the host crystal structure?
Going back to Gentry's early research (Gentry, 1968, 1971; Gentry, et al., 1973), it is apparent that the association of concentric colored haloes with polonium is actually speculative. Gentry adopts and expands on the work of Joly (1917) that polonium isotopes were the most likely cause of the features observed. Joly did most of his work with discoloration haloes in the first decade of the Twentieth Century, a time when the structure of the atom was just being discovered, and before the crystal structure of minerals had been unraveled. This was also the period when the nature of radioactivity was just being uncovered. Joly made the very speculative assumption that if alpha particles could travel 3-7 centimeters in air, then they would only travel 1/2000 of that distance in biotite mica. From this generalization, and without considering the variability in the density and the crystal structure of the host mica (or even the variable density of air), Joly attempted to correlate the radial size of the concentric ring haloes with the alpha particles of specific isotopes (he was first to suggest polonium). He also tried to develop an age dating technique based on the diameter of the halo features - the larger the halo, the longer the radiation had been affecting the host mineral grain. Henderson (1939) carried Joly's work further, developing a classification scheme for the different patterns of discoloration haloes he observed, and deriving hypotheses for how short-lived polonium could find its way into the host crystal structure.
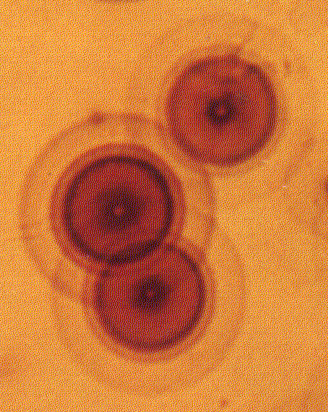
Figure 2. Concentric haloes in biotite mica considered by Gentry to be caused by polonium isotope decay (Gentry, 1992).
In his research, Gentry followed Joly's approach of defining an idealized model based on the average distance traveled in air by alpha particles of different energy. He then measured concentric ring haloes in mica (or fluorite, or cordierite) to see which ones matched his model. Of course, the large assumption here is that his model is correct.
How can alpha particle emissions result in discrete colored rings? Gentry (1992) provides the explanation "that alpha particles do the most damage at the end of their paths." This would appear to be a reference to the “Bragg Effect”, the phenomenon whereby charged particles lose energy during penetration of different media. When charged particles (a proton or an alpha particle) pass through matter, they lose energy primarily by ionizing the atoms of material being passed through. The amount of energy required to ionize an atom depends on the specific element involved. In general, the lower the energy of the impacting charged particle, the faster it loses energy. Another way of looking at this is - as the particle loses energy, it slows down, and as it slows down, it interacts more strongly with surrounding atoms, causing it to decelerate even more rapidly. Finally, the particle loses all of its kinetic energy and comes to rest, at which time it can capture electrons and become a neutral atom. The amount of energy loss - and thus disruption of the affected medium - is greatest at the end of the particle’s path of travel (although energy will have been given up, and ionization of surrounding atoms will have occurred, along the entire path). For protons, with a single charge and relatively low mass, this effect is extremely pronounced, and is the basis for proton beam treatment of various tumors. Beams of high energy protons can be adjusted to have almost all of their energy loss (the Bragg Peak) occur within a small volume of cancerous tissue, with almost no energy deposition in the healthy tissue beyond. The effect is less dramatic for alpha particles with their larger mass and double charge. Also, energy loss is a statistical phenomenon; a spread of energies always results after a beam of monoenergetic charged particles has passed through a given thickness of an absorbing medium - known as energy straggling (Knoll, 1979). Gentry’s own attempts to duplicate alpha particle damage in minerals using a helium ion beam illustrates these effects. In these experiments, the ion beam intensity was adjusted to produce a discoloration pattern in the irradiated mineral (the extent of the discoloration being then compared to the halo diameter associated with each alpha-decay energy). An ion beam irradiates an "area" and has luminosities (particles per beam cross section per unit time) many orders of magnitude higher than the "spherical" volumetric emission of alpha particles from radioactive centers in mineral grains. Short exposure to an ion beam can create damage patterns equivalent to millions of years of low-level natural alpha exposure. Gentry (1974) notes the problem of beam intensity required to achieve a specific level of discoloration. The pattern produced by Gentry through ion beam bombardment was a zone of discoloration, faintest near the source, and increasing in intensity up to a relatively sharp termination - exactly what would be predicted for the Bragg Effect in minerals. Gentry's ion-beam work, however, was not able to produce multiple bands or the sharply defined concentric ring structure of certain halos, suggesting that such features may have another cause separate from alpha particle damage.
Even if the spherical damage halos are the result of alpha particle decay, Gentry's assignment of alpha particle energies to specific halo ring sizes is highly questionable. To understand why this is so, imagine a hollow sphere. You can pass an infinite number of 2-dimensional planes through the sphere, and each will result in a circular cross-section. Only those planes that intersect the center of the sphere, however, will result in a circular cross-section which has the same diameter as the sphere itself. All other circular cross-sections will be of smaller diameters than the sphere. For Gentry to be able to state that he can consistently correlate alpha particle energy with a given circular halo size, he has to be able to demonstrate that he is measuring the maximum spherical diameter of the halo in question. Given that many of the spherical diameters assigned to specific radioisotope decays differ by only a small amount, missing the maximum diameter of the spherical halo even slightly will result in a erroneous assignment of energy to ring diameter. Nowhere in Gentry's writings does he demonstrate how he is able to assure that his thin sections intersected each halo in an equatorial plane (i.e., the maximum halo diameter).
An additional consequence of this uncertainty in finding the maximum halo diameter relates to spherical damage halos that contain a central mineral inclusion that is small relative to the size of the halo. Thin sections which cut the spherical halo at a distance from the center will result in a concentric ring pattern which doesn't appear to have a central radiation source for the rings - when there actually is one.
Gentry (1970, 1974), himself, notes a number of aspects about concentric haloes which cannot be explained by the alpha decay hypothesis. Dwarf and giant haloes cannot be reconciled with any known alpha decay energies. Gentry postulates that these anomalous size haloes represent new elements or new forms of alpha decay. Neither explanation seems likely given the current state of knowledge of radioactive elements (ICRP, 1983; Parrington, et al., 1996). Other haloes show "ghost" rings which don't correspond to any measured alpha decay energy, and which remain unexplained. Finally, there are "reversed coloration" haloes, supposed uranium haloes in which the gradation of color intensity in the circular band is opposite to, and the ring diameters offset from, those in a "normal" uranium pattern. Other exceptions to Gentry's energy vs. ring diameter model have been noted by Odom and Rink (1989) and Moazed et al. (1973). Gentry speculates on the cause(s) of some of these anomalous features, but provides no empirical data to support any explanation. Indeed, Gentry appears to be more willing to question the evidence provided by the physical samples than to question the validity of his model.
Perhaps the most damaging challenge to Gentry's hypothesis comes not from what has been observed, but from what is missing. Of the three major, naturally occurring radioactive elements, uranium, thorium, and potassium, two - uranium and thorium - are marked by decay series involving alpha particle emissions. Gentry's polonium haloes are attributed to alpha particle decay of the polonium isotopes Po-210, Po-214, and Po-218, all part of the uranium-238 decay chain. Thorium-232 decays to stable Lead-208 through a series of steps which include two additional polonium isotopes, Po-212 and Po-216. Thorium has an elemental abundance between three and four times that of uranium in the Earth's crust. Also, in areas of uranium enrichment, such as those from which Gentry's halo samples apparently have come, thorium is also enriched. These thorium decay series polonium isotopes have alpha decay energies well within the range documented for uranium-series polonium decay. Thus, polonium isotopes which result from the decay of naturally occurring thorium-232 should also produce characteristic haloes. In fact, according to Gentry's model, all polonium isotopes should be represented equally. However as Collins (1997) points out, Gentry has identified only halos for those isotopes of polonium associated with the decay of uranium-238; halos attributable to polonium-212 and polonium-216 are not found. Additionally, haloes attributable to the two polonium isotopes in the decay series of uranium-235 (Po-211 and Po-215) are also missing. Uranium-235 comprises 0.71% of naturally occurring uranium (uranium-238 makes up 99.3%).
If concentric rings haloes aren't caused by alpha particles, what causes them? Both Joly (1917) and Gentry (1992) discounted the possibility that beta particles may play a role in coloration changes within minerals; however, neither author gives a basis for this rejection beyond the erroneous statement that beta particle energies are too low to have any affect. High energy beta particles have the well documented ability to break molecular bonds. Combinations of alpha and beta decay particles, beta particles alone, or some completely non-radioactive process may be the cause of the observed mineral discoloration haloes.
Odom and Rink (1989) examined giant radiohaloes in mica and proposed an alternative hypothesis for their formation. They compare the circular halo structures in mica with radiation-induced color halos (RICHs) in quartz. In the quartz crystalline structure, aluminum can occasionally substitute for a silicon atom, creating a slight charge imbalance. Alpha particles from uranium decay create hole-trapping centers around the aluminum atoms. This in turn creates a semi-conductive area where beta particles (also resulting from uranium decay) can cause diffusion and discoloration over a fairly large area. The width of the resulting halo can be correlated with migration of valence-band holes along a radiation-induced charge potential in the host crystal. While this is an attractive hypothesis, Odom and Rink cautiously note that the crystal structures and chemical composition of quartz and mica are significantly different. Quartz is known to have natural piezoelectric properties missing in the mica group minerals. Without further investigation, haloes caused by migrating hole trapping centers is speculative for minerals other than quartz.
3) If the concentric haloes are indeed caused by alpha radiation damage, is polonium decay the only possible cause?
Even if we assume that concentric ring haloes actually are due to alpha radiation damage, an immediate problem arises with the short half-life of the polonium isotopes themselves. In order to leave a visible radiation damage halo, the affected mica or fluorite crystals would have to crystallize before the polonium decayed away to background levels - about 10 half lives. For polonium isotopes, this correlates to between a fraction of a second (Po-212, Po-214, Po-215) and 138.4 days (Po-210). Gentry's hypothesis calls for pure, concentrated polonium at the center of each ring. The model makes no distinction between which polonium isotopes should be present - thus, there should be equal likelihood for all. He points out that there is no known geochemical process by which such concentrations can occur during crystallization of a magma, concluding therefore that polonium haloes are indicative of some non-natural or supernatural occurrence.
An alternative possibility is explored by Brawley (1992) and Collins (1997). They note that many concentric ring haloes line up along visible fractures within the host mica. Such fractures are very common in mica crystals. Micro-fractures could provide conduits for the rapid movement and concentration of radon-222, a gaseous daughter product of uranium-238 which forms part way along the decay chain leading to polonium. Radon-222, itself an alpha emitter, has a half life of 3.82 days and is continuously produced in the decay of the parent uranium. Migration of radon along fractures with hold-up points at tiny structural traps would result in exactly the same concentric ring pattern assigned by Gentry to polonium alone (because polonium is a daughter isotope of radon decay). A distinct radon halo will not necessarily be identified s the radon alpha decay energy is very close to that of polonium-210 and the two ring structures commonly cannot be distinguished (Moazed, et al., 1973).
The radon hypothesis is more attractive than Gentry's model as it doesn't require supernatural occurrences. The development of fractures in the grains of mica after crystallization has occurred, and the migration of radon along these fractures over the course of millennia, is much more in keeping with current geologic models of rock formation.
| Expanding on the Radon migration idea
While Gentry does not provide a conclusive argument for demonstrating the relationship between concentric haloes and Polonium decay, the contribution of alpha-decay to halo development cannot be discounted entirely either. Collins (1997) reports that concentric ring halo structures commonly line up along visible micro-fractures in the host mineral grains, implying some association of the haloes with the fractures. An interesting argument can be developed to support the idea that concentric ring haloes are created following the migration of radon gas along mineral fractures.
Polonium isotopes are produced in each of the radioactive decay chains of naturally occurring uranium-238, thorium-232, and uranium-235.
Gentry's studies identify concentric ring structures correlated with each of the three polonium isotopes in the uranium-238 decay series. Ring haloes correlated with polonium isotopes from the uranium-235 or the thorium-232 decay series are not reported, although they would have to be present under Gentry's primordial origin hypothesis.
The first polonium isotope in each decay series is the daughter of a different radon atom; these radon precursors have greatly different half-lives.
If polonium ring structures are the result of radon migration along micro-fractures (Collins' hypothesis), then the half-life of the specific radon precursor is important. Clearly, radon-222 can migrate much further than the other two radon species before it decays away. Also, because of its significantly longer half-life, radon-222 can accumulate in more significant concentrations in structural traps along the micro-fracture surfaces. Under these circumstances, one would expect to see many more radiogenic ring haloes associated with uranium-238 series polonium isotopes than those of the other two decay chains.
This explanation is more consistent with what is observed than Gentry's hypothesis, and is completely consistent with the standard geological model for rock formation. |
|---|
4) Is Gentry's hypothesis consistent with, or explain all other evidence pointing to a great age for the Earth?
Gentry's hypothesis quickly runs into trouble with all of the accumulated evidence from many fields of earth science pointing conclusively to a great age for the Earth. Not the least of these evidences is radiometric age dating. To reconcile his presumed young age for the Earth with reported isotopic age dates for rocks around the world, Gentry (1992) argues that radioactive decay rates have varied over time. He is forced to conclude that decay rates for polonium have remained constant while those of dozens of other radioactive isotopes were many orders of magnitude greater 6,000 to 10,000 years ago. This of course gives rise to several major inconsistencies:
The decay rate and the energy of emitted alpha particles are both related to the imbalance of neutrons and protons in an atomic nucleus, and are controlled by the strong nuclear force and the binding energy for the particular nuclide. Anything more than a fractional change in the decay rate over time would require variation in the fundamental forces of nature and the relationship of matter and energy. There is no evidence that anything of the sort has ever occurred.
There are many independent lines of reasoning beside radiometric age dating for concluding that the Earth is far older than 6,000 years. Other geologic processes, with completely independent mechanisms, which demonstrate a long period for Earth history include:
Literally hundreds of other examples could also be presented.
Gentry recognizes this as a problem and, in addition to his variable decay rate concept, calls upon several other lines of reasoning and “evidence” in an attempt to support his Young Earth model. One such line of reasoning involves the decay of naturally occurring uranium isotopes (U-238 and U-235) in the mineral zircon into their final daughter lead isotopes (Pb-206 and Pb-207). Gentry posits that lead is lost readily over time because it fits poorly into the crystal structure of zircon. Gentry, et al., 1982, examined zircons from a granite dated at 1.5 billion years old. They applied a generalized diffusion model and, using measured values, showed that lead should be highly retained in zircon crystals over a temperatures range of 100 -313 ° C. In his Po-halo paper, Gentry appears to be referring to this earlier study when he states: "...calculations show that 50 micron-size zircons taken from the bottom of the drill hole (313° C) should have lost 1% of their lead content in about 300,000 years." From this calculation he concludes that if the granite is really as old as 1.5 bilion years, nearly all of the radiogenic lead should have disappeared by this time. Instead, laboratory analyses actually showed a high degree of lead retention in the zircon sample. Therefore, Gentry concludes that the host granite must really be of a very young age.
If the granite host of the zircon crystals is really old, as other measurements suggest, and the lead isotopes haven’t disappeared, how could Gentry’s prediction be so far off the mark.? The answer is really quite simple. Gentry’s research team in 1982 was looking at the ability of manmade crystalline substances - SYNROCK - to encapsulate nuclear waste. For this study, they used an idealized model of uniform diffusion out of a diffusing medium. The type of medium to which this equation applies most accurately is an amorphous solid such as a gel or a glass. The only time zircon comes close to this condition is when there has been severe radiation damage to the mineral’s crystal lattice - a relatively uncommon occurrence (and very detectable with microscopic examination). In reality, zircon is one of the most durable of crystalline solids, resistant to both chemical attack and mechanical abrasion. It also resists radiation damage.
Gentry and his team used this idealized diffusion model for several reasons. First, it is simpler to calculate a diffusion rate when you don’’t have to deal with the complications of a crystalline lattice. Secondly, for an evaluation of the effectiveness of nuclear waste encapsulation it is preferable to ask "what is the worst possible performance we might experience?" The model used by Gentry in1982 was just such a "worst-case" analysis as it presents the most rapid diffusion situation. The addition of a substantial amount of highly radioactive isotopes to a material such as Synrock would likely result in extensive damage to the crystalline structure of the material - and thus, treating the material as an idealized diffusing medium is an appropriate conservatism. However, this was not the appropriate formula for describing the behavior of natural zircon containing very low concentrations of uranium and thorium. It is not surprising, therefore, that the measured ratios of 206Pb/207Pb did not match the predictions. Once again, Gentry used the wrong predictive model.
A 1997 study by Lee and others, set out to directly measure the diffusion of uranium, thorium, and lead out of natural zircon crystals under carefully controlled laboratory conditions. Their results showed that at temperatures around 1,100° C, lead diffuses about 4 orders of magnitude faster than uranium or thorium. They also showed that the closure temperature for zircon is greater than 900° C. What this means is, at temperatures in the range evaluated by Gentry, et al., 1982, little to no Pb diffusion would be expected - exactly what was measured.
No one disputes that diffusion of daughter isotopes can and does occur during the natural history of a rock body. For this reason, geochronologists have developed the concordia - discordia method of analyzing uranium - lead isotopic ratios, and the lead - lead isochron method of age dating. The concordia - discordia method allows an assessment not only of the degree of radiogenic lead loss, but also can be used to determine when the major period of lead loss occurred. The lead - lead isochron method, by comparing the amount of radiogenic lead daughters to the non-radiogenic lead component of a sample, also compensates for the possibility of radiogenic lead loss over time. There are several good texts on radiometric dating which explain these techniques in detail (e.g., Dalrymple, 1991).
Gentry presents a similar "model" for helium retention in granitic rocks (remember, a helium atom is the same as an alpha particle that is produced by radioactive decay). According to this model, helium, a gas, should rapidly diffuse out of a crystal structure. Thus, when higher than predicted levels of helium retention are measured, the presumption is that the rock is of a young age. Once again, however, it is the model that is questionable. In reality, retention of helium in zircons is not unexpected. Once uranium reaches equilibrium with its daughter products (approximately 1 million years), helium production assumes a steady state. At this point, helium retention/loss will most likely be controlled solely by temperature - consistent with Gentry's own measurements. A better test would be to determine the helium content of zircons from a number of granites of different ages and sample depths to see what patterns emerge.
Summary/Conclusions
Gentry's polonium halo hypothesis for a young Earth fails all tests. Gentry's entire thesis is built on a compounded set of assumptions. He is unable to demonstrate that concentric haloes in mica are caused uniquely by alpha particles resulting from the decay of polonium isotopes. His samples are not from "primordial" pieces of the Earth's original crust, but from rocks which have been extensively reworked. Finally, his hypothesis cannot accommodate the many alternative lines of evidence that demonstrate a great age for the Earth. Gentry rationalizes any evidence which contradicts his hypothesis by proposing three "singularities" - one time divine interventions - over the past 6000 years. Of course, supernatural events and processes fall outside the realm of scientific investigations to address. As with the idea of variable radioactive decay rates, once Gentry moves beyond the realm of physical laws, his arguments fail to have any scientific usefulness. If divine action is necessary to fit the halo hypothesis into some consistent model of Earth history, why waste all that time trying to argue about the origins of the haloes based on current scientific theory? This is where most Creationist arguments break down when they try to adopt the language and trappings of science. Trying to prove a religious premise is itself an act of faith, not science.
In the end, Gentry's young Earth proposal, based on years of measuring discoloration haloes, is nothing more than a high-tech version of the Creationist "Omphalos" argument. This is the late nineteenth century proposition that while God created the Earth just 6,000 years ago according to the Genesis account, He made everything appear old. Unfortunately, because Gentry has published his original work on haloes in reputable scientific journals, a number of basic geology and mineralogy text books still state that microscopic discoloration haloes in mica are the result of polonium decay.
Footnote: Omphalos means navel, and is the title of a book by Phillip Grosse. He argued that God created Adam and Eve with navels even though they had not developed in a womb.
References:
Brawley, John, 1992, "Evolution's Tiny Violences: The Po-Halo Mystery: An Amateur Scientist Examines Pegmatitic Biotite Mica", Talk.Origins Archive, www.talkorigins.org/faqs/po-halos/violences.html.
Collins, Lorence G., 1997, "Polonium Halos and Myrmekite in Pegmatite and Granite," www.csun.edu/~vcgeo005/revised8.htm, 9 pgs.
Collins, Lorence G., 1999, "Equal Time for the Origin of Granite - a Miracle," Reports of the National Center for Science Education, Volume 19, No. 2, pp. 20-28.
Dalrymple, G. Brent, 1991, The Age of the Earth, Stanford University press.
Gentry, Robert V., 1968, "Fossil Alpha-Recoil Analysis of Certain Variant Radioactive halos" Science, Vol. 160, p. 1228-1230.
Gentry, Robert V., 1970, "Giant Radioactive Halos: Indicators of Unknown Radioactivity," Science, Vol. 169, pp. 670-673
Gentry, Robert V., 1971, "Radiohalos: Some Unique Lead Isotope Ratios and Unknown Alpha Radioactivity," Science, Vol. 173, p. 727-731.
Gentry, Robert V., S.S. Christy, J.F. McLaughlin, J. A. McHugh, 1973, Nature, Vol. 244, p. 282.
Gentry, Robert V., 1974, "Radioactive Halos in a Radiochronological and Cosmological Perspective", Science, Vol. 184, pp. 62-66.
Gentry, Robert V., Warner H. Christie, David H. Smith, J.F. Emery, S.A. Reynolds, and Raymond Walker, 1976, "Radiohalos in Coalified Wood: New Evidence Relating to the Time of Uranium Introduction and Coalification," Science, Vol. 194, pp.315-318
Gentry, Robert V., T.J. Sworski, H.S. McKown, D.H. Smith, R.E. Eby, W.H. Christie, 1982, "Differential Lead Retention in Zircons: Implications for Nuclear Waste Containment,"Science, Vol. 216, p. 296-298.
Gentry, Robert V., 1992, Creation's Tiny Mystery, Earth Science Associates, Knowville, TN, 3rd Edition.
Henderson, G.H., 1939, A quantitatve study of pleochroic halos, V. the genesis of halos, Royal Society of London, Proceedings, Series A, v. 173, p. 250-264.
Hyndman, Donald W., 1985, Petrology of Igneous and Metamorphic Rocks, 2nd Edition, McGraw-Hill, N.Y., p. 75.
ICRP, 1983, Radionuclide Transformations, International Commission on Radiological Protection, Publication 38, Pergamon Press, New York, NY, 1250p.
Joly, J., 1917, The genesis of pleochroic halos, Philosophical Transactions of the Royal Society of London, Series A, v. 217, p. 51.
Knoll, Glenn F., 1979, Radiation Detection and Measurement, John Wiley and Sons, New York, NY
Lee, James K, Ian S. Williams, and David J. Ellis, 1997, "Pb, U, and Th diffusion in natural zircon", Nature, Vol. 390, pp. 159-162.
Moazed, Cyrus; Richard M. Spector; Richard F. Ward, 1973, Polonium Radiohalos: An Alternate Interpretation, Science, Vol. 180, pp. 1272-1274.
Odom, L.A., and Rink, W.J., 1989, "Giant Radiation-Induced Color Halos in Quartz: Solution to a Riddle," Science, v. 246, pp. 107-109.
Parrington, Josef R., Harold D. Knox, Susan L. Breneman, Edward M. Baum, Frank Feiner, 1996, Nuclides and Isotopes: Chart of the Nuclides, 15th Edition, General Electric Co. and KAPL, Inc.
Taylor, S. Ross, and McLennan, Scott, 1996, "The Evolution of the Continental Crust," Scientific American, January, 1996.
Wakefield, J. Richard , 1988, Geology of Gentry's "Tiny Mystery", Journal of Geological Education, May, 1988.